Answer:
The temperature of 1.25 moles of an ideal gas if it occupies 20.0 liters at a pressure of 1.45 atm is 9.58 °C
Step-by-step explanation:
The complete question is with options, as follow:
"What is the temperature of 1.25 moles of an ideal gas if it occupies 20.0 liters at a pressure of 1.45 atm?
9.58°C
17.2°C
23.2°C
36.3°C"
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables as follows:
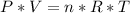
where:
- P = pressure
- V = volume
- n = number of moles
- R = gas constant, whose value depends on the units you use
- T = temperature
In this case, the values you know are:
- P=1.45 atm
- V= 20 L
- n= 1.25 moles
- R= 0.08205746
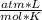
Replacing:
1.45 atm*20 L= 1.25 moles*0.08205746
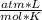 *T
*T
Solving, you get:
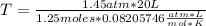
T=282.73 °K
Being 0 ° C = 273.15 ° K, then:
T=282.73 °K=9.58 °C
The temperature of 1.25 moles of an ideal gas if it occupies 20.0 liters at a pressure of 1.45 atm is 9.58 °C