Step 1 - Understanding the relation between temperature and volume
As we have seen in the previous exercise, pressure, volume and temperature can all modify the state of a gas sample, are there are proportionality relations between them.
When pressure is kept constant, the volume increases as the temperature increases. We can state it mathematically as:
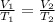
We'll be using this expression to solve the exercise.
Step 2 - Using the formula to solve the exercise
The exercise asks us to find T2. We also know that:
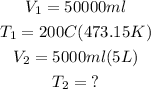
Always remember to work with the temperature in K. The proportionality between volume and temeprature will only work if the temperature is expressed in K.
Substituting these values in the equation above:
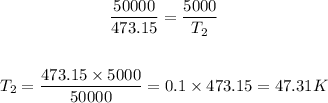
Step 3 - Converting K back to °C
To convert K to °C, we just need to subtract 273.15:
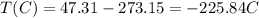
The new temperature is thus -225.84°C.