Answer:
The number of molecules is 3.07x10^23.
Step-by-step explanation:
1st) From the molar mass of NH4OH (35.04g/mol) we can calculate the number of moles in 18g of NH4OH:
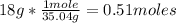
2nd) With the Avogadro's number (6.022x10^23 molecules/mol) we can convert the moles into molecules:
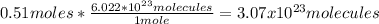
So, the number of molecules is 3.07x10^23.