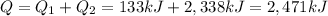Answer: 2471 kJ
Step-by-step explanation:
The problem can be split into two steps:
1- Calculate the heat energy needed to raise the temperature of the ice from -9 C to 0 C
2- Calculate the heat energy required to melt the ice
Let's solve them separately.
1- The heat energy needed to raise the temperature of the ice from -9 C to 0 C is given by:
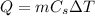
where
m=7 kg is the mass of the ice
Cs=2108 J/kgK is the specific heat capacity of ice
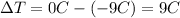 is the temperature gain
is the temperature gain
Substituting into the formula,
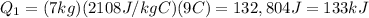
2- the heat energy required to melt the ice is given by

where
m=7 kg is the mass of the ice
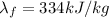 is the specific latent heat of fusion for ice
is the specific latent heat of fusion for ice
Substituting into the formula,
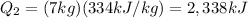
3- So, the total heat energy needed for the entire process is