Answer: The correct answer is 5.
Step-by-step explanation:
Valence electrons are the number of electrons that are present in the last shell of an element. It is judged by looking at the electronic configuration.
The number of electrons in an element is the atomic number of that element.
Nitrogen is the 7th element in the periodic table with atomic number 7.
Number of electrons in nitrogen = 7
Electronic configuration of nitrogen =
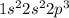
From the above configuration, the valence electrons in nitrogen is 5 because the last shell has n = 2.
Therefore, the correct answer is 5.