Answer: The incorrect statement is all acid names end with -ic.
Step-by-step explanation:
Acids are defined as the chemical species which donate hydrogen ions when dissolved in water.
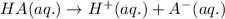
The nomenclature of the acids that are derived from the anions attached to hydrogen ion:
- If the name of the anion attached ends with -ate, then the prefix of the acid is -ic. For Example:
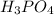 , the anion is phosphate and the name of the acid is phosphoric acid.
, the anion is phosphate and the name of the acid is phosphoric acid. - If the name of the anion attached ends with -ite, then the prefix of the acid is -ous. For Example:
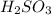 , the anion is sulfite and the name of the acid is sulfurous acid.
, the anion is sulfite and the name of the acid is sulfurous acid. - If the name of the anion attached ends with -ide, then the prefix of the acid is -ic. For Example: HCl, the anion is chloride and the name of the acid is hydrochloric acid.
Hence, the incorrect statement is all acid names end with -ic.