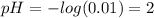Answer:
pH of solution is 2
Step-by-step explanation:
HCl is a strong acid. Hence it gets completely dissociated in aqueous solution to produce
![[H_(3)O^(+)]and Cl^(-)](https://img.qammunity.org/2018/formulas/chemistry/middle-school/kdch2xjpcmzg9waz643t02glcyd63tkrqh.png) .
.
Chemical equation related to dissociation of HCl is given as-
HCl+H_{2}O\rightarrow [H_{3}O^{+}]+Cl^{-}
pH of a solution is a tool to measure acidity. It is defined as-
![pH=-log[H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/rb4w9dn06rm7czwtyq9rq103ott2tpb205.png)
where
![[H^(+)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/tixcvmiul05vtrn8s7qstptt69zx1u6brh.png) represents concentration of
represents concentration of
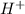 in molarity.
in molarity.
In aqueous solution, all
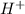 gets converted into
gets converted into
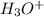 .
.
Hence, in aqueous solution,
![pH=-log[H_(3)O^(+)]](https://img.qammunity.org/2018/formulas/chemistry/middle-school/d57eu3eo9de2lrckra7ck6yoqitp5y8i8l.png)
Here
![[H^(+)]=0.01M](https://img.qammunity.org/2018/formulas/chemistry/middle-school/log7anma0kkylj9934ezmlllp1m0ezqq43.png) . So
. So
![[H_(3)O^(+)]=0.01M](https://img.qammunity.org/2018/formulas/chemistry/middle-school/cqzxewwnfbpkkczvyf8ekqmc777b7mcef6.png)
Hence