Given, equilibrium constant Kp = 0.0870
Pressure of PCl₃ = 0.50 atm
Pressure of Cl₂ = 0.50 atm
Pressure of PCl₅ = 0.20 atm
Reaction quotient, Q =
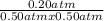
Q = 0.8
Q > K, net reaction is to the left
Q= K, no net reaction
Q < K, net reaction is to the right
Q = 0.8 and Kp = 0.0870
Q is greater than K, so the net reaction is to the left or to the reactant side.
Therefore, the reaction must proceed to the left or to the reactant side in order to reach equilibrium.