The first thing to do is to calculate the number of moles in the given molecules. For this, we use the Avogadro number which tells us that one mole of any substance contains 6.022x10^23 molecules.
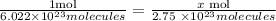
We clear x,
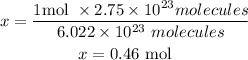
Now we will use the molar weight of chlorine to determine the mass in 0.46 moles.
Molar weight of chlorine = 35.453 g/mol

So, the mass in 2.75x10^26 molecules of Chlorine is 16.20g