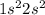Answer:
C is equal to 2 and D is equal to 2.
Step-by-step explanation:
Here C and D represents number of electrons in 1s and 2s orbitals.
Be has 4 electrons. Now, in order write electronic configuration of an atom, electron filling should be started from orbital with lowest energy.
Energy order of orbital with increasing energy: 1s< 2s< 2p< 3s< 3p< 4s< 3d....
So, filling of electron should start from 1s orbital then 2s, 2p, 3s, 3p and so on.
Each orbital can have maximum of 2 electrons.
So electronic configuration of Be is: