Answer:
Electronic configuration represents the total number of electrons that a neutral element contains. We add all the superscripts to know the number of electrons in an atom. The electrons are filled according to Afbau's rule in order of increasing energies.
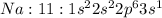
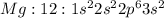
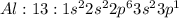
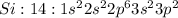
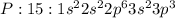
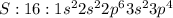
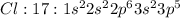
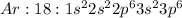
The last electron enters the third shell in all elements from Sodium to Argon.