Answer: The given electronic configuration is long hand notation.
Step-by-step explanation:
Long-hand notation of representing electronic configuration is defined as the arrangement of total number of electrons that are present in an element.
Noble-gas notation of representing electronic configuration is defined as the arrangement of valence electrons in the element. The core electrons are represented as the previous noble gas of the element that is considered.
The given electronic configuration of potassium (K):
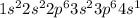
The above configuration has all the electrons that are contained in the nucleus of an element. Thus, this configuration is a long-hand notation.