Answer : The amount of energy released are, -36836.8 J
Solution :
Formula used :
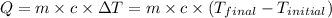
where,
Q = heat or energy released or absorbed = ?
m = mass of water = 40 g
c = specific heat of water =
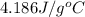
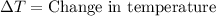
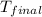 = final temperature =
= final temperature =
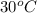
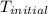 = initial temperature =
= initial temperature =
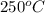
Now put all the given values in the above formula, we get
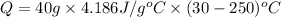
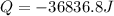
The negative energy means energy is released and positive energy means energy is absorbed.
Therefore, the amount of energy released are, -36836.8 J