The first thing to do is to dry the salt at a moderate temperature, about 40 to 50°C. A little more than 10 grams of NaCl is weighed and every few minutes it is weighed again until there is no significant change in weight. This is done because the salt may contain water and the precision of the procedure would be reduced, so the water should be removed.
Then we weigh the 10 grams of salt on a balance.
We measure 500 mL of water, preferably in a volumetric balloon for greater precision.
We mix both components and shake the mixture to dilute well the salt (Solute) in the water. The sodium (Na) and chlorine (Cl) atoms, initially bound together in the form of a crystal, are dissolved by the water molecules. Water is a solvent. In the end, we will have a homogeneous mixture, i.e. we can only see one phase in spite of having two substances.
The molarity of the mixture is calculated with the following equation:
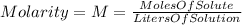
So, we need to calculate the moles of NaCl. We will use the molar mass of NaCl.
Molar mass of NaCl = 58.44g/mol
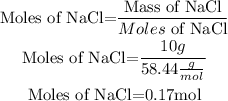
Volumen of solution= 500mL = 0.5L
So, the molarity will be:
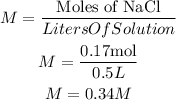
The molarity of this mix will be 0.34M, and we will have a homogeneous mixture.