Answer: The combination of two grey elements will lead to the formation of molecule of element.
Step-by-step explanation:
Element is defined as the substance having similar kind of atoms.
Compound is defined as the substance having different kind of atoms.
Molecules of elements are defined as the substances which are made up of similar type of atoms. For Example:
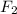 etc..
etc..
Molecules of compounds are defined as the substances which are made up of different type of atoms. For Example:
 etc..
etc..
We are given:
Two grey elements are combining to form a molecule. The molecule formed is known as molecule of element because 2 same kind of elements are combining.
Hence, this will lead to the formation of molecule of element.