Answer:
On both the side chlorine atoms will be present in equal numbers
Explanation:
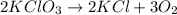
Number of atoms on left hand side:
Potassium atoms, K = 2 × 1 = 2
Chlorine atoms, Cl = 2 × 1 = 2
Oxygen atom, O= 2 × 3 =6
Number of atoms on right hand side:
Potassium atoms, K = 2 × 1 = 2
Chlorine atoms, Cl = 2 × 1 = 2
Oxygen atom, O= 3 × 2 =6
From the atomic inventory , number of chlorine atoms on both the sides are equal as per as Law of Conservation of mass.