Answer: The correct answer is particles that struck the center of the atom were repelled.
Step-by-step explanation:
Rutherford gave an experiment which is known as gold foil experiment.
In his experiment, he took a gold foil and bombarded it with the alpha particles (carrying positive charge). These alpha particles are also known as helium nucleus. It is represented as
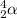 .
.
He thought that these particles will pass straight through the foil, but to his surprise, some of them deflected their path and a few of them bounced back.
From this he concluded that in an atom, a small positive charge in the center is present. Due to this positive charge, the alpha particles deflected their path and some of them bounced straight back from their path.
Hence, the correct answer is particles that struck the center of the atom were repelled.