Answer:
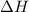 for the reaction is 300kJ.
for the reaction is 300kJ.
Step-by-step explanation:
By convention:
Energy gained or absorbed is taken as positive and energy released is taken as negative.
In the question,
Reactant A + 2D is absorbing energy of amount 800kJ to reach an intermediate state and at first releasing 200 kJ of energy and then releasing 300 kJ of energy. Thus, total of 500 kJ of energy is released.
So,
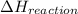 = Energy gained - Energy lost
= Energy gained - Energy lost
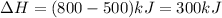
Hence,
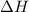 for the reaction is 300kJ.
for the reaction is 300kJ.