Answer : The correct option is, between a metal and a nonmetal that share electrons
Explanation :
Covalent compound : These are the compounds in which atoms are bonded covalently. Covalent bonds are formed by the equal sharing of electrons. These bonds are commonly formed between two non-metals.
For example :
 is a covalent compound in which carbon and oxygen both are non-metal.
is a covalent compound in which carbon and oxygen both are non-metal.
Ionic compound : These are the compounds in which atoms are bonded through ionic bond. Ionic bonds are formed by the complete transfer of electrons. The ionic bonds are formed between one metal and one non-metal.
For example :
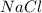 is an ionic compound in which sodium is a metal and chlorine is a non-metal.
is an ionic compound in which sodium is a metal and chlorine is a non-metal.
Hence, the correct option is, between a metal and a nonmetal that share electrons