Answer:
- Increasing the temperature.
- Increasing the reactants' initial concentrations.
- Using a catalyst.
Step-by-step explanation:
Hello,
In this case, this is about the combustion of methane which is shown below:
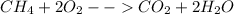
Therefore, three ways to speed up this chemical reaction is via:
- Increasing the temperature: in this fashion, it is possible to accelerate the molecules crashing (via kinetic energy) in order to diminish the activation energy and to allow the reaction to be carried our more rapidly.
- Increasing methane's and oxygen's initial concentration: it is known that the reaction rate depends directly upon the reactants initial concentration, thus, by increasing the reactants' initial concentration, the molecules will crash closely and there will be more possibilities for them to crash and turn into the products.
- Using a catalyst: it is widely known that catalysts diminish the reaction rate, in this manned a catalyst could be used to speed the reaction up.
Best regards.