Answer:
Step-by-step explanation:
a) Before we proceed to write the cathode and anode half reactions, we have to identify the anode and the cathode
The cathode is connected to negative terminal, while anode is connected to the positive terminal
Reduction i.e electron gain happens at the cathode while oxidation i.e electron loss happens at the anode
From the set-up that we have, copper is the cathode, while gold is the anode
We have the half cell reactions as follows:
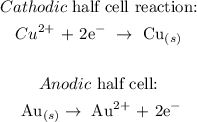
b) We want to get the theoretical minimum voltage
We calculate that as follows:
![undefined]()