Answer: Yes,they will have the same mass.
Step-by-step explanation:
According to law of conservation of mass:
'Mass can neither be destroyed nor be created during a chemical reaction'
In a balanced chemical reaction :
- Total numbers of atoms on the reactants side are always equal to the total numbers of atoms on the product side.
- Total mass of the atoms on the reactant side of the equation is always equal to the total mass of atoms on the product side.
For example:
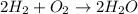
Number of atoms on reactant side =
=(2)2 Hydrogen atoms + (1)2 Oxygen atoms = 6 atoms
Number of atoms on the product side =
=(2)((2 Hydrogen atoms) + (1 Oxygen atoms)) = 6 atoms
Mass of of reactants = (2)(number of hydrogen atoms)(mass of hydrogen atom) + (1)(number of oxygen atoms)(mass of oxygen atoms)
= (2)(2)(1u)+(1)(2)(16u) = 36u
Mass of products = 2((number of hydrogen atoms)(mass of hydrogen atom)+(number of oxygen atoms)(mass of oxygen atom))
= 2((2)(1u)+(1)(16u)) = 36u
Mass of of reactants = Mass of products = 36u