Answer: Option (B) is the correct answer.
Step-by-step explanation:
When two different liquids of different boiling point are mixed and boiled together then liquid with lower boiling point will change into vapors readily. These vapors upon cooling will be collected and hence separated out in a separate container.
This process of separation is known as distillation.
For example, a solution containing diethyl ether (boiling point
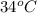 and dioxane (boiling point
and dioxane (boiling point
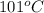 ) can be separated out by distillation.
) can be separated out by distillation.
Thus, we can conclude that distillation uses boiling points to separate different liquids.