Answer: Option (b) is the correct answer.
Step-by-step explanation:
When a neutral element loses an electron then it acquires a positive charge and it is known as a cation.
For example, when a neutral iron atom loses three electrons then it forms a
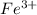 ion.
ion.
On the other hand, when a neutral element gains an electron then it acquires a negative charge and it is known as a anion.
For example, a sulfate ion means
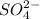 ion.
ion.
Thus, we can conclude that out of the given options sulfate is not a cation.