Step-by-step explanation:
When a neutral atom loses an electron then it develops a positive charge and results into the formation of a cation.
For example, Na is an alkali metal with atomic number 11 and its electronic distribution is 2, 8, 1.
So, in order to become stable it loses its 1 valence electron and thus is forms
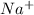 ion.
ion.
Thus, we can conclude that the element sodium (Na) has an atomic number of 11 with one outer valence electron, this element would likely form a cation.