Answer : The number of electrons present in 3d subshell of Mn are, 5 electrons.
Explanation :
Electronic configuration : It is defined as the representation of electrons around the nucleus of an atom.
Number of electrons in an atom are determined by the electronic configuration.
The given element is 'Mn' that has 25 number of electrons.
The electronic configuration of Mn will be,
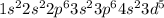
From the electronic configuration of 'Mn' we conclude that, there are 5 number of electrons present in 3d subshell of Mn.
Hence, number of electrons present in 3d subshell of Mn are, 5 electrons.