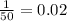Answer:
0.02
Step-by-step explanation:
The balanced equation is
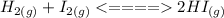
So the equilbrium constant is calculated the following way
![([HI]^(2) )/([H_(2] )[I_(2)]) = 50\\](https://img.qammunity.org/2018/formulas/chemistry/high-school/snvsl6s00nuvizrg4z8invcwnzbmyfltnu.png)
When the reaction is written in reverse
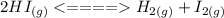
Then the equilibrium constant becomes:
![([H_(2) ][I_(2) ])/([HI]^(2) )](https://img.qammunity.org/2018/formulas/chemistry/high-school/xep93a4yg1kshk3np4krzlp7lbdrmd5wfc.png)
which the reciprocal of K eq in the first part. So to find Keq of the second form the equation we also reciprocate 50