Answer: The number of formula units in the given amount of sodium oxide is

Step-by-step explanation:
Formula units are defined as lowest whole number ratio of ions in an ionic compound. It is calculate by multiplying the number of moles by Avogadro's number which is
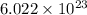
We are given:
Number of moles of sodium oxide = 0.25 moles
Number of formula units =

Hence, the number of formula units in the given amount of sodium oxide is
