When we use an equation for stoichiometry, it has to be balanced.
The given equation:
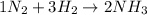
Is already balanced, because there are 2 N on both the left and right sides and 6 H on both the left and the right sides.
So, we can skip the balancing.
Now, we can use the coefficients on the involved compounds to calculate the quantity we want.
We have:
Number of moles of H₂: ?
Coefficient of H₂: 3
Number of moles of NH₃: 5 mol
Coefficient of NH₃: 2
So, we can apply the rule of three:
H₂ ---- NH₃
x ---- 5 mol
3 ---- 2
So, we can write the relation:
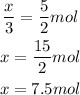
So, we need 7.5 mol of hydrogen gas.