Answer: The amount of glucose present in 175 mL of solution is 9.625 grams.
Step-by-step explanation:
We are given:
5.50 % (m/v) glucose solution. This means that 5.50 grams of glucose is present in 100 mL of volume.
To calculate the amount of glucose in 175 mL of volume, we apply unitary method, we get:
In 100 mL of volume, the amount of glucose present is 5.50 g.
So, in 175 mL of volume, the amount of glucose present is
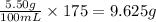
Hence, the amount of glucose present in 175 mL of solution is 9.625 grams.