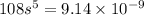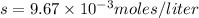Answer:
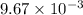 moles /liter
moles /liter
Step-by-step explanation: The equation for the reaction will be as follows:
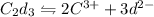
1 mole of
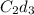 gives 2 moles of
gives 2 moles of
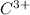 and 3 moles of
and 3 moles of
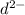 .
.
Thus if solubility of
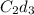 is s moles/liter, solubility of
is s moles/liter, solubility of
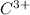 is 2s moles\liter and solubility of
is 2s moles\liter and solubility of
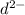 is 3s moles/liter
is 3s moles/liter
Therefore,
![K_sp=[C^(3+)]^2[d^(2-)^3]](https://img.qammunity.org/2018/formulas/chemistry/college/yoy6ia7dferdm0ecvttndbajv9v91k243a.png)
![9.14* 10^(-9)=[2s]^2[3s]^3](https://img.qammunity.org/2018/formulas/chemistry/college/jcym5t4he0m8pfaiazkxf26t1hq3dpqa0h.png)