Answer: Number of moles of ions in 0.716 L of 0.450 M solution is 1.2888 moles.
Solution:
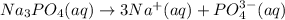
Volume of the solution 716 mL
Molarity of the solution : 0.450 M
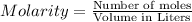
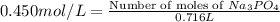
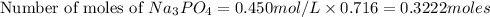
According to reaction one mol
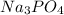 is giving 4 moles of ions.
is giving 4 moles of ions.
Number of moles of ions in 0.716 L of 0.450 M solution is =
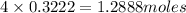
Hence,number of moles of ions in 0.716 L of 0.450 M solution is 1.2888 moles.