Answer:- Kc = 6.63
Solution:- First given equation is:
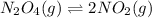
The equilibrium expression for this equation is written as:
![Kc=([NO_2]^2)/([N_2O_4])](https://img.qammunity.org/2018/formulas/chemistry/middle-school/okyctq975m4ix9afw8novut0q24tx2o6mp.png)
The second given equation is:
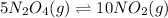
The equilibrium expression for this equation is written as:
![Kc^'=([NO_2]^1^0)/([N_2O_4]^5)](https://img.qammunity.org/2018/formulas/chemistry/middle-school/ymcez4nag9t4x8lt3u0lw815sx262onfhr.png)
Comparing these two expressions, to get this second expression from the first one, we need to do the fifth power of first equilibrium expression.
Hence,

Plug in the value of Kc in it from first expression,
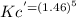
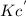 = 6.63
= 6.63
So, the Kc of second equation is 6.63.