Answer: It is named as nitric acid in aqueous solution.
Step-by-step explanation:
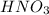 is an acid because it releases hydrogen ions when dissolved in water. The chemical equation for the ionization of
is an acid because it releases hydrogen ions when dissolved in water. The chemical equation for the ionization of
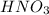 follows:
follows:
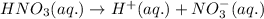
When an element is present in the higher oxidation state in a compound, a suffix '-ic' is added at the end.
When an element is present in the lowest oxidation state in a compound, a suffix '-ous' is added at the end.
Nitrogen shows oxidation state of +5 and +3. In the given compound, nitrogen is present in +5 oxidation state. Thus, in the naming of the compound, a suffix '-ic' is added.
Hence, the given compound is named as nitric acid in aqueous solution.