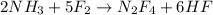Answer : The balanced chemical reaction will be,
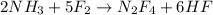
Explanation :
Balanced chemical reaction : It is defined as the chemical reaction in which the number of individual atoms of an element in reactant side always be equal to the number of individual atoms of an element in product side.
The given balanced reaction will be,
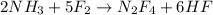
By the stoichiometry we can say that, 2 moles of
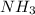 react with 5 moles of
react with 5 moles of
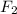 to give 1 mole of
to give 1 mole of
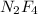 and 6 moles of
and 6 moles of
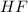 as a product.
as a product.
Hence, the balanced chemical reaction will be,