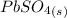Answer:
Lead (II) sulphate,
Step-by-step explanation:
A precipitate reaction is one involving two aqueous reactants in which one of the products is a solid or a precipitate. It can also be defined as a reaction involving the formation of insoluble salt from mixture of two soluble salts.
The equation of reaction involving
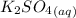 and
and
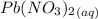 is as follows:
is as follows:

The precipitate formed in this case is
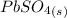 because it is the only insoluble one of the two products.
because it is the only insoluble one of the two products.
Hence the white color precipitate that forms is