Answer:
The stoichiometric coefficient of the antimony(III) oxide is 5.
Step-by-step explanation:
The compound
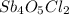 is prepared by heating the antimony(III) oxide with antimony(III) chloride.
is prepared by heating the antimony(III) oxide with antimony(III) chloride.
The balanced chemical reaction is given by:
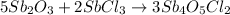
The stoichiometric coefficient of the antimony(III) oxide is 5.