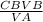What we are give: Concentration of base (CB) = 3.4 ×
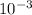
Then convert all volume in ml to L.
Volume of base (VB) 25.0ml = 0.025L
Volume of acid (VA) 16.6ml = 0.0166L
Now that we have everything we use the formula CAVA=CBVB.
Make 'CA' the subject then solve.
CA=