Answer:
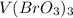
Step-by-step explanation:
Hello,
In this case, vanadium is specified with +3 as its oxidation state, however it is stated that vanadium is combined with the bromate ion, which according to the IUPAC rules, accounts for bromine's last oxidation state, that is +5, in such a way, we build up the bromate ion forming the bromic acid shown below:
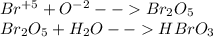
Such bromate comes from the bromic acid's anion, this is
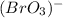 , thus, the required formula of the vanadium (III) bromate turns out into:
, thus, the required formula of the vanadium (III) bromate turns out into:
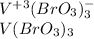
Best regards.