In order to find the new pressure, we can use the relation below:
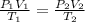
Where P is the pressure, V is the volume and T is the temperature.
If the temperature remains the same and the volume increases four times, we have T1 = T2 = T and V2 = 4V1:
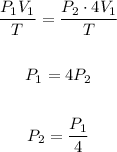
We can see that the new pressure is 4 times lower than the initial pressure.
Therefore the correct option is 1).