Step-by-step explanation:
Alkaline earth metals are the metals which are present in group 2 of the periodic table.
Elements of this group are beryllium, magnesium, calcium, strontium, barium and radium.
So, barium is an alkaline earth metal and its electronic configuration is
![[Xe]6s^(2)](https://img.qammunity.org/2018/formulas/chemistry/high-school/rpeh00vvrrfmezjamucyigwx8uh3js1m27.png) .
.
It contains two valence electrons. Therefore, in order to attain noble gas configuration it needs to lose 2 electrons.
Hence, on losing two electrons it forms
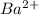 ion.
ion.
Thus, we can conclude that barium have to give up 2 electrons to achieve a noble-gas electron configuration.