Answer : The hybridization of carbon in
 is,
is,
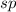 hybridized.
hybridized.
Explanation :
As we know that when a carbon attached to the four single sigma bonds then the hybridization will be,
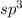 .
.
When a carbon attached to the three single sigma bonds and one-
 bond that means a carbon bonded to another atom by the double bond then the hybridization will be,
bond that means a carbon bonded to another atom by the double bond then the hybridization will be,
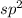 .
.
When a carbon attached to the two single sigma bonds and two-
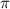 bonds that means a carbon bonded to another atom by the triple bond then the hybridization will be,
bonds that means a carbon bonded to another atom by the triple bond then the hybridization will be,
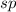 .
.
As per question, the given molecule is,
 . In this molecule, a triple bond is present between the nitrogen and carbon atom and a single bond is present between the carbon and oxygen atom. That means, a carbon bonded to another atom by the triple bond then the hybridization will be,
. In this molecule, a triple bond is present between the nitrogen and carbon atom and a single bond is present between the carbon and oxygen atom. That means, a carbon bonded to another atom by the triple bond then the hybridization will be,
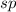 .
.
Hence, the hybridization of carbon in
 is,
is,
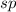 hybridized.
hybridized.