Answer:
109.5∘ and 109.5∘
Step-by-step explanation:
The chemical compound known as methylhydrazine with the formula
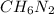 has a total number of 9 atoms which include 1 carbon atom, 2 nitrogen atoms, and 6 hydrogen atoms. The molecular geometry of the chemical compound is trigonal pyramidal. Due to the molecular geometry of this compound, the approximate values of the C-N-N and H-N-H bond angles are 109.5∘ and 109.5∘.
has a total number of 9 atoms which include 1 carbon atom, 2 nitrogen atoms, and 6 hydrogen atoms. The molecular geometry of the chemical compound is trigonal pyramidal. Due to the molecular geometry of this compound, the approximate values of the C-N-N and H-N-H bond angles are 109.5∘ and 109.5∘.