Answer: 702.2 mmHg
Explanation: According to Dalton's law of partial pressure, the total pressure is the sum of partial pressures of individual components.
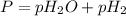
P=total pressure
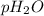 = partial pressure of water
= partial pressure of water
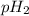 = partial pressure of hydrogen
= partial pressure of hydrogen
partial pressure of
 at 30°C is 31.8 mmHg
at 30°C is 31.8 mmHg
734 mmHg = 31.8 mmHg +
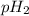
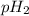 = 702.2 mmHg
= 702.2 mmHg