Answer:
24.86 g
Step-by-step explanation:
To answer this problem we need to look up the atomic weight of lead in the periodic table: 207.2.
With that information we know that 1 mol of lead weighs 207.2 grams, so we can calculate the weight of 0.12 mol of lead:
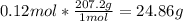
So the mass of the fishing weight is 24.86 g