Hello!
How many molecules of nitrogen dioxide are in 1.28 g of nitrogen dioxide?
Let's first find the molecular mass of nitrogen dioxide, knowing that by Avogadro's Law for each mole of a substance we have 6.02 * 10²³ molecules.
N = 1*14 = 14 amu
O = 2*16 = 32 amu
---------------------------
molecular mass of nitrogen dioxide = 14 + 32 = 46 g/mol
How many molecules of nitrogen dioxide are in 1.28 g of nitrogen dioxide NO2?
46 g ---------------- 6.02*10²³ molecules
1.28 g ------------- y molecules
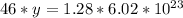
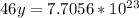
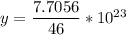
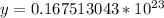
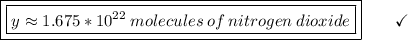
________________________
I Hope this helps, greetings ... Dexteright02! =)