Answer: Oxygen
Explanation:
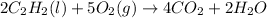
As can be seen from the balanced chemical equation, 2 moles of acetylene react with 5 moles of oxygen.
Thus 37 moles of acetylene will react with
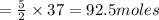 of oxygen.
of oxygen.
But
 available is only 81.0 moles.
available is only 81.0 moles.
Thus now we take it the other way:
5 moles of oxygen react with 2 moles of acetylene
81 moles of oxygen will react with
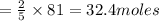 of acetylene.
of acetylene.
Limiting reagent is the reagent which limits the formation of product. Excess reagent is one which is in excess and thus remains unreacted.
Thus oxygen is the limiting reagent and acetylene is the excess reagent as (37-32.4)= 4.6 moles of acetylene are left.