Answer:
![K_c=([HbO_2])/([Hb][O_2])](https://img.qammunity.org/2018/formulas/chemistry/college/zztvwnok4uzex0ixih1ropahgwdy3wtn19.png)
Step-by-step explanation:
Equilibrium constant is the ratio of the concentration of products to the concentration of reactants each term raised to its stochiometric coefficients.
For a general reaction:
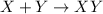
![K_c=([XY])/([X][Y])](https://img.qammunity.org/2018/formulas/chemistry/college/4swzkrywrc8wwbjxvs9fj52tnavs0d3lfp.png)
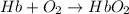
The equilibrium constant in terms of concentration is written as :
![K_c=([HbO_2])/([Hb][O_2])](https://img.qammunity.org/2018/formulas/chemistry/college/zztvwnok4uzex0ixih1ropahgwdy3wtn19.png)