The sodium chloride is NaCl in the equation, while the sodium carbonate is the Na₂CO₃.
The stoichiometry of them in this reaction is 1 Na₂CO₃ to 2 NaCl.
So, first we need to convert the 27.2g of Na₂CO₃ to number of moles of Na₂CO₃. We do that by using the molar mass of Na₂CO₃:
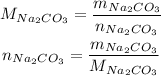
The molar mass of Na₂CO₃ can be calculated using the molar masses of Na, C and O, which can be consulted in a periodic table:

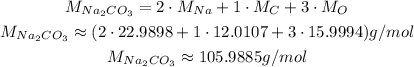
So, the number of moles of Na₂CO₃ is:
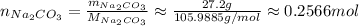
Now, since the stoichimetry is 1 Na₂CO₃ to 2 NaCl, each mol of Na₂CO₃ will produce 2 moles of NaCl, thus:
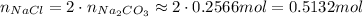
Now, we use the molar mass of NaCl to calculate the mass of it:

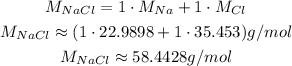
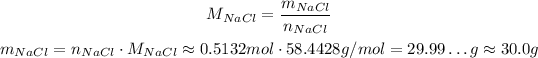
So, the mass of sodium chloride that can be produced from 27.2g of sodium carbonate is approximately 30.0g.