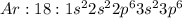Answer: Sodium: D) Malleable, soft, and shiny
Silicon: B) Has properties of both metals and nonmetals
Bromine: C) Highly reactive gas
Argon: A) Nonreactive gas
Explanation: Sodium is a metal as it can lose it's valence electron easily so as to attain stable configuration.
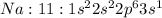
Metals are soft, malleable and shiny.
Silicon is a metalloid which has characteristics between those of metals and non metals. It has 4 valence electrons thus it can neither easily lose like metals nor easily gain like non metals.
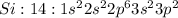
Bromine is a gas which is very reactive as it only needs one electron to attain stable electron configuration.
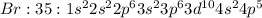
Argon is a noble gas which is non reactive as it has stable configuration and thus neither lose nor gain electrons.